See the difference that DUOBRII Lotion can make over time2
Photos have not been retouched. Individual results may vary.
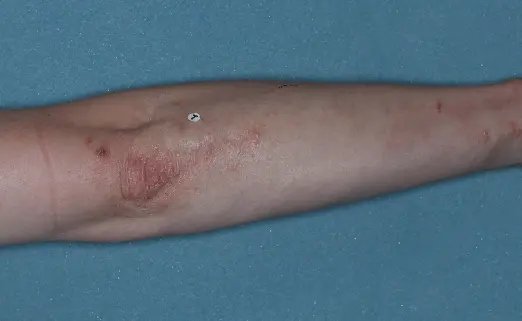
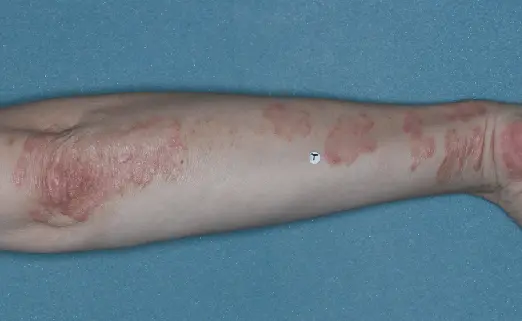
Before (Baseline)
After (8 weeks)
Photos have not been retouched. Individual results may vary.
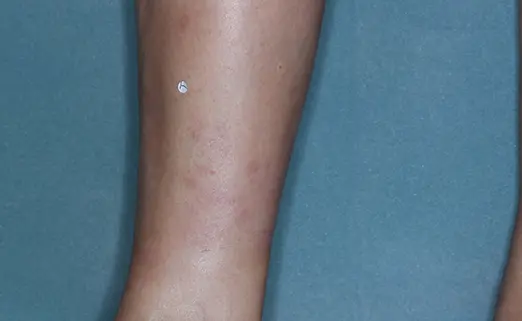
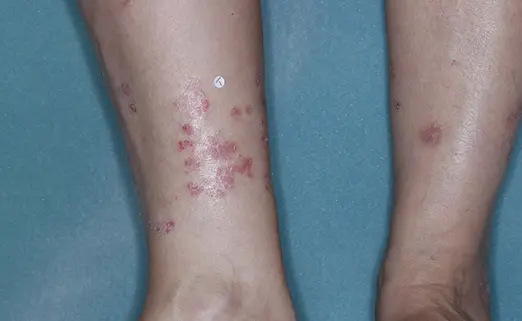
Before (Baseline)
After (8 weeks)
Photos have not been retouched. Individual results may vary.
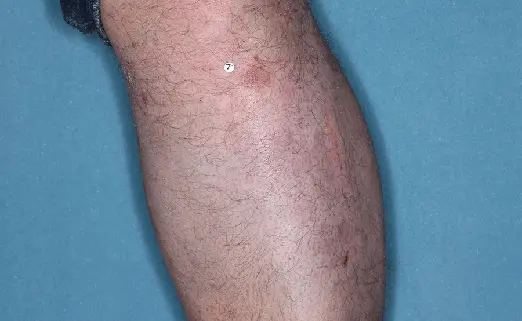
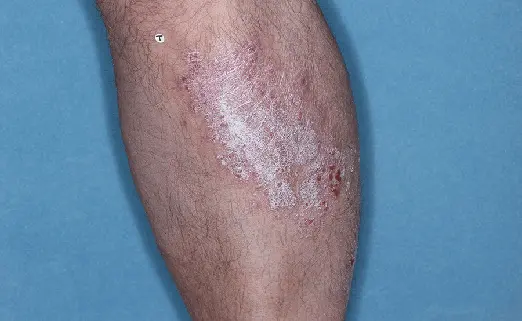
Before (Baseline)
After (8 weeks)
Photos have not been retouched. Individual results may vary.
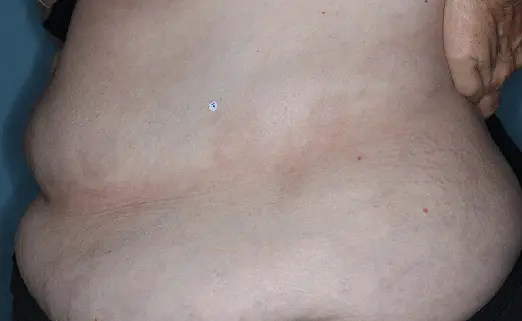
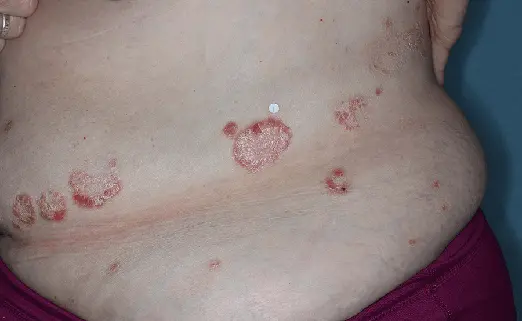
Before (Baseline)
After (8 weeks)
Photographs of patients were taken during 2 prospective, multicenter, randomized, double-blind, vehicle-controlled phase 3 clinical trials in 418 patients with moderate-to-severe plaque psoriasis. Patients were treated with DUOBRII Lotion or vehicle, applied once daily. The primary endpoint was treatment success at 8 weeks based on IGA. Secondary endpoints evaluated treatment success at 2, 4, 6, and 12 weeks.2,3