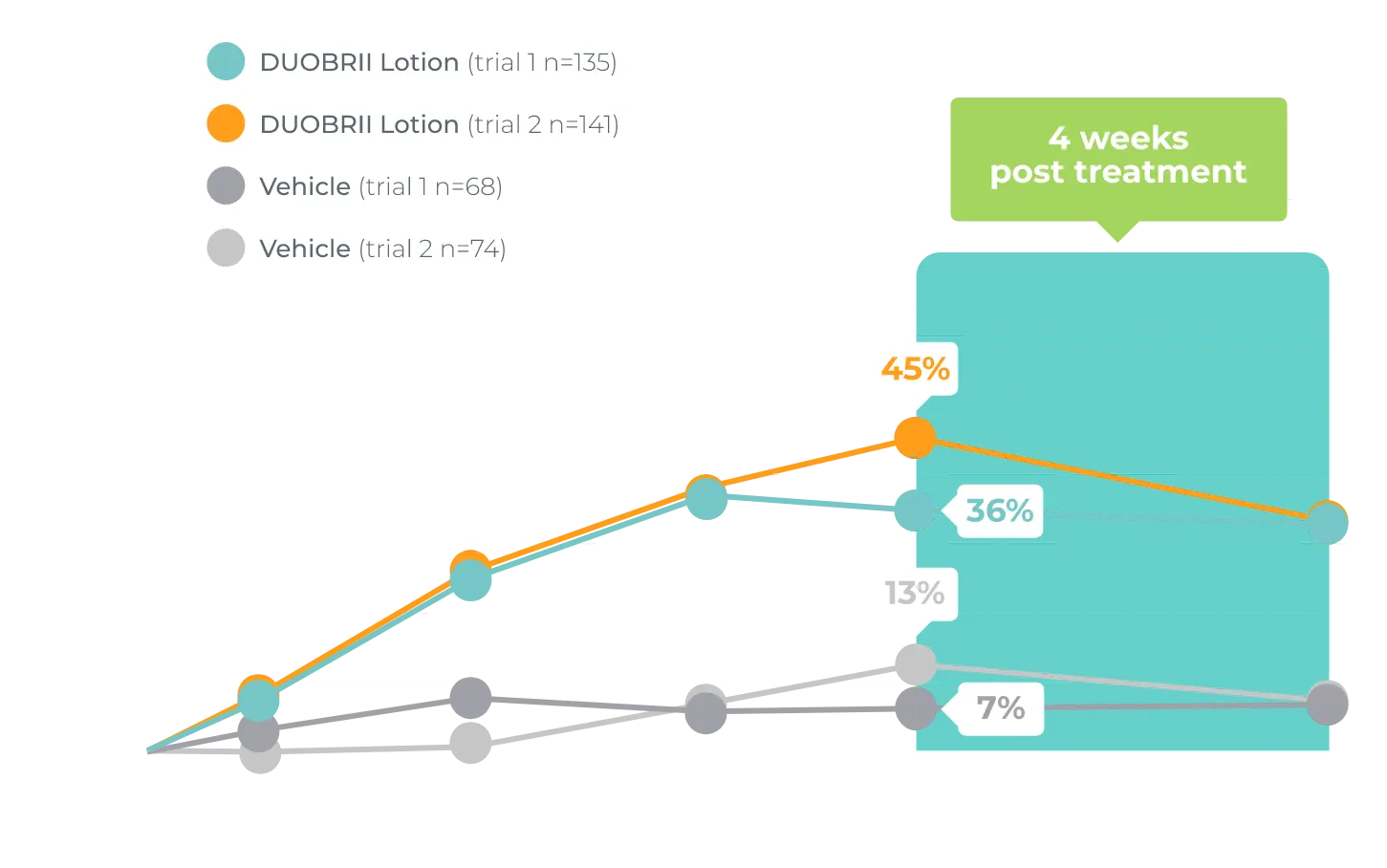
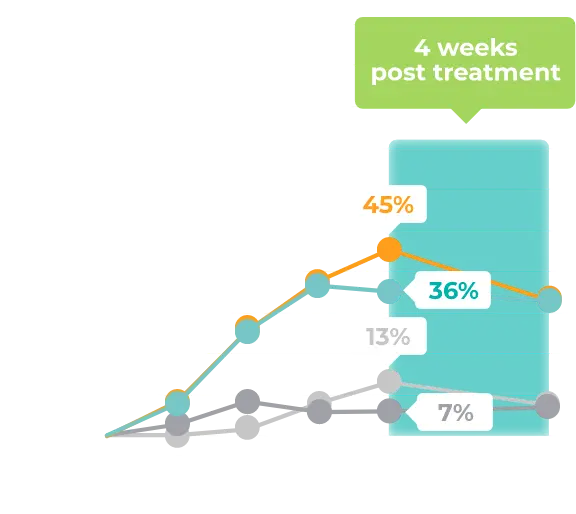
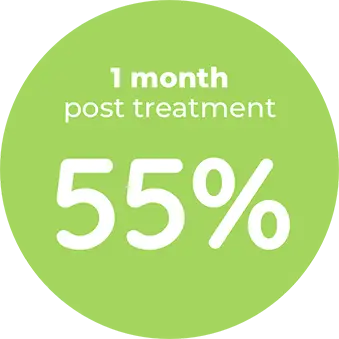
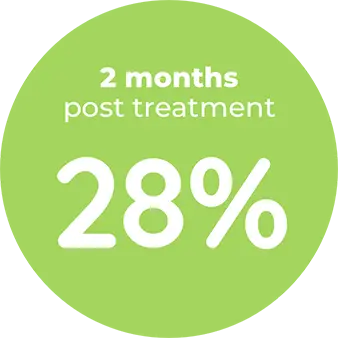
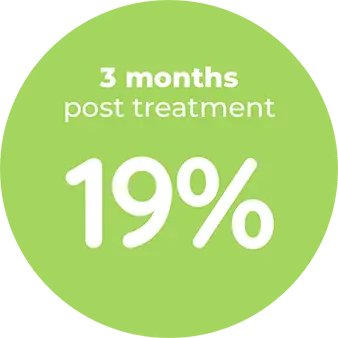
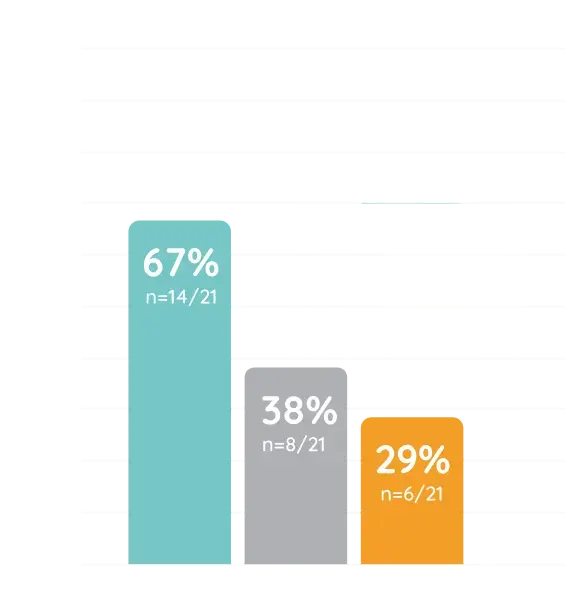
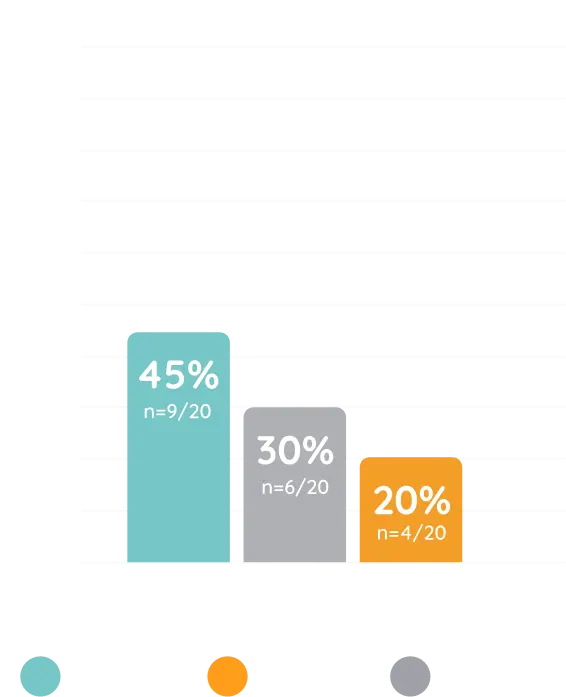
Contraindication
DUOBRII Lotion is contraindicated in pregnancy.
Warnings and Precautions
- Embryofetal risks. Women of child-bearing potential should be warned of the potential risk of fetal harm from DUOBRII and use adequate birth-control during treatment with DUOBRII. A negative result for pregnancy should be obtained within 2 weeks prior to treatment. If the patient becomes pregnant during treatment, discontinue DUOBRII Lotion and advise patient of the potential hazard to the fetus.
Contraindication
DUOBRII Lotion is contraindicated in pregnancy.
Warnings and Precautions
- Embryofetal risks. Women of child-bearing